Seminar 16th February 2010 3:30 p.m. 53/4025
Quantum Chemical Topology: a route to next generation force fields?
Professor Paul Popelier
Manchester Interdisciplinary Biocentre (MIB) and School of Chemistry, University of Manchester
- Categories
- Biomedical, C, Complex Systems, Density functional Theory, Fortran, Materials, Molecular Dynamics, Optimisation, Structural biology
- Submitter
- Chris-Kriton Skylaris
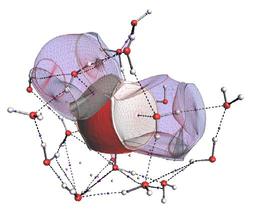
Representation of three water molecules in liquid water according to Quantum Chemical Topology. The central water (non?transparent) is flanked by two semi?transparent neighbours.
15:30 Coffee, tea and biscuits
16:00 Start of seminar
Abstract
Including polarisation in force field potentials is universally accepted as important. In 2008 a whole issue of J.Chem.Theor & Comput. was dedicated to this topic, presenting modifications to existing polarization schemes. Here we propose a very rent approach, which is closer to quantum mechanical reality and which avoids ad hoc models, such as the Drude oscillator, for example. We take advantage of the present abundance of (small scale) computing power to model polarisation directly and “literally”, as the full change in an atom’s electron density. In the proposed method we use machine learning to capture how atomic multipole moments change in response to changing positions of neighbouring atoms. The atoms are malleable boxes of finite volume. This method covers polarisation in a very direct way, valid at short?range. It treats charge transfer in a unified way alongside dipolar and higher?rank polarisation. Quantum Chemical Topology (QCT) partitions molecular systems (e.g. water clusters) into finite atoms, which can be reassembled to form molecules in clusters. We present results on water clusters obtained by Multilayer Perceptrons (MLP), Radial Basis Function Neural Nets (RBFNN) and Kriging. This method can also handle intramolecular interactions. Although it is tested on amino acids first.
References [1] M.G.Darley, C.M.Handley and P.L.A. Popelier, J.Chem.Theory & Comp., 4, 1435 (2008). [2] C.M.Handley and P.L.A. Popelier, J.Chem.Theor. & Comp., 5, 1474?1489 (2009). [3] C.M.Handley, G.I.Hawe, D.B.Kell and P.L.A. Popelier, Phys.Chem.Chem.Phys. 11, 6365 (2009).