Multi-objective design optimisation of coronary stents
- Started
- 1st October 2008
- Research Team
- Sanjay Pant
- Investigators
- Neil Bressloff, Georges Limbert
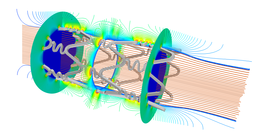
Velocity streamlines and drug concentration contours inside a stented coronary artery. The expanded geometry of the stent-artery assembly is obtained by a finte element modelling of the stent balloon-expansion in a representative diseased coronary artery.
Coronary stents are tubular type scaffolds that are deployed, using an inflatable balloon on a catheter, most commonly to recover the lumen size of narrowed (diseased) arterial segments. Their presence, however, can lead to adverse biological responses – in-stent restenosis, formation of neointima within 12 months of the implant, and thrombosis, formation of a blood clot inside the artery. Currently, an “ideal stent” - that recovers arterial shape with no adverse response - does not exist, even though, as a multi-billion dollar industry, stent design has witnessed a fairly rapid evolution from bare metal stents of increasing complexity, through shape memory alloy stents, polymer coated, drug eluting stents to biodegradable stents made from polymers or corrodible metals.
The adverse responses are likely to be multi-factorial but their causes are not completely understood. However, there are studies implying a correlation between the stent design, which is a common differentiating factor between the numerous stent designs available in the market today, and the adverse responses. There are several properties in an ideal stent design that are desirable. For instance, it should have high radial strength to provide good arterial support post expansion, have high flexibility for easy manoeuvrability during deployment, cause minimal injury to the artery when being expanded, and, for drug eluting stents, should provide a uniform drug distribution in the arterial tissue. Often, with any stent design, these objectives are in competition such that improvement in one objective is a result of trade-off in others. This project aims to evaluate the performance of stents on each of the aforementioned aspects, and use the results to perform optimization studies on a parametric representation of stent designs.
Various models to evaluate a stent’s performance are developed. Finite element analysis (FEA) is first used to model the stent expansion process, in a representative diseased artery, and performance parameters such as structural strength and arterial injury are quantified. Thereafter, on the expanded geometry obtained from the expansion simulations, pulsatile blood flow and drug release simulations, using computational fluid dynamics, are performed to quantify haemodynamic alteration and drug-distribution. Also, a separate FEA model to quantify flexibility is developed. Once the analysis models are set up, a technique to parameterise the geometry of stent is developed. Using this parameterization and the aforementioned models, surrogate modelling (kriging) is used to emulate the physical response of the stents in the parameter space. Finally, a non-dominated sorting genetic algorithm (NSGA-II) is used to search the surrogate models for the optimal family of stent designs, which minimise the features that lead to adverse biological responses in stented arteries.
Categories
Life sciences simulation: Biomedical
Physical Systems and Engineering simulation: Biomechanics, CFD, Structural dynamics
Algorithms and computational methods: Evolutionary Algorithms, Finite elements, Finite volume, Optimisation
Simulation software: Abaqus, Star CCM+
Programming languages and libraries: Java, Matlab, Python